-
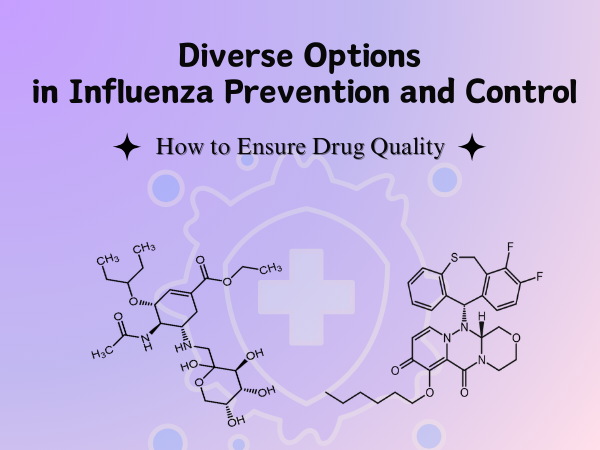
Diverse Options in Influenza Prevention and Control: How to Ensure Drug ...
As the flu season persists, drug quality control becomes a critical link in ensuring public medication safety. Drug impurity reference standards, serving as the "benchmark" in drug quality research,...
See More
-
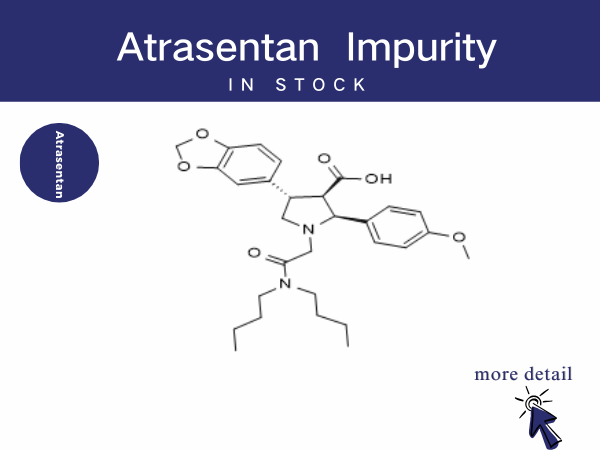
Atrasentan Impurities—A Critical Quality Control Factor in IgA Nephro...
In August 2025, atrasentan hydrochloride tablets were officially approved by China‘s National Medical Products Administration (NMPA) for the reduction of proteinuria in adult patients wi...
See More
-
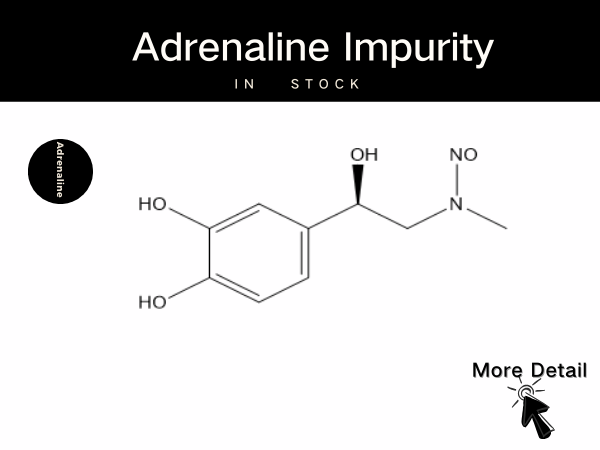
Full Set In Stock | Adrenaline Impurities Now Shining Bright!...
SZEB specializes in supplying Adrenaline impurity reference standards, including the N-NitrosoAdrenaline series and Adrenaline EP impurity series, to meet diverse experimental needs. Thes...
See More
-
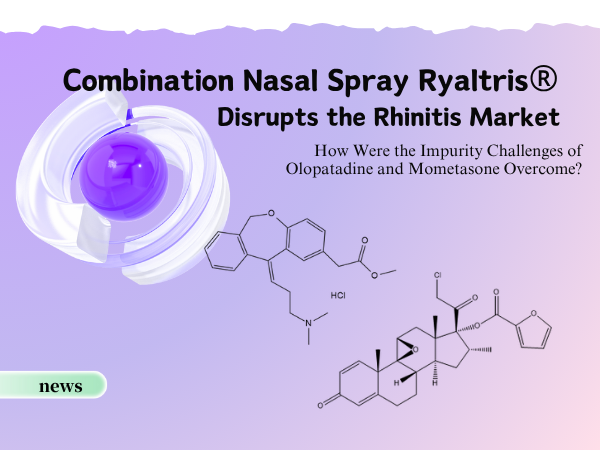
Combination Nasal Spray Ryaltris® Disrupts the Rhinitis Market: How We...
On November 10th, the compound nasal spray containing Olopatadine Hydrochloride and Mometasone Furoate (brand name: Ryaltris®), jointly developed by Grand Pharmaceutical Group‘s subsidiary...
See More
-
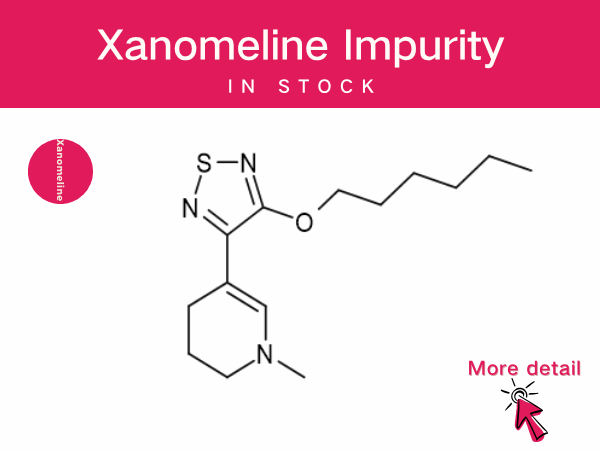
Xanomeline : A Pioneering Non-Dopaminergic Therapy...
The fixed-dose combination drug Cobenfy (Kar XT), composed of xanomeline and trospium chloride, received FDA approval in September 2024 for the treatment of schizophrenia in adults. This mar...
See More
-
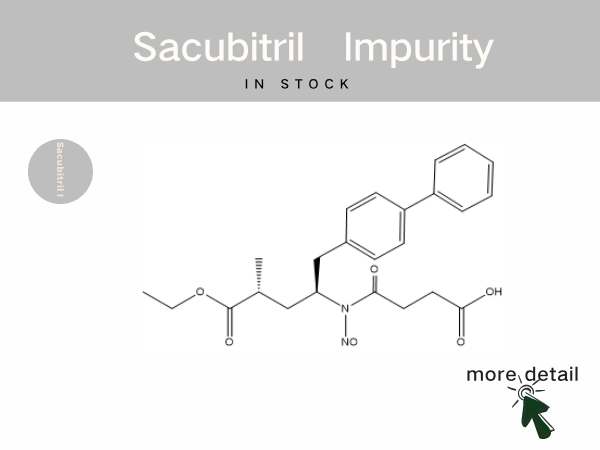
In Stock | Sacubitril impurity references...
Sacubitril Valsartan Sodium Tablets is the world‘s first commercially available angiotensin receptor-neprilysin inhibitor (ARNI). It was approved for marketing in 2017 by the National Medi...
See More
-
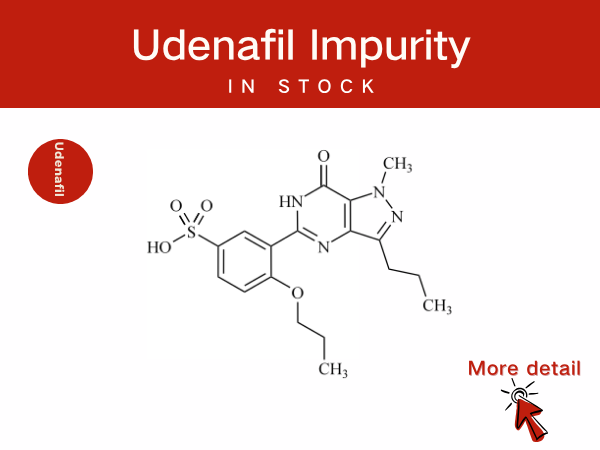
Udenafil Impurity Control and Scientific Innovation: Professional Pharmac...
Udenafil is a selective phosphodiesterase type 5 (PDE5) inhibitor. It is primarily used for the treatment of male erectile dysfunction (ED) and is also applied in the management of premature ej...
See More
-
China’s First Domestically Developed Dulaglutide Injection Approved for ...
On August 8, Boan Biotechnology announced that its independently developed Boyouping® (Dulaglutide Injection) has received market approval from the National Medical Products Administration...
See More
-
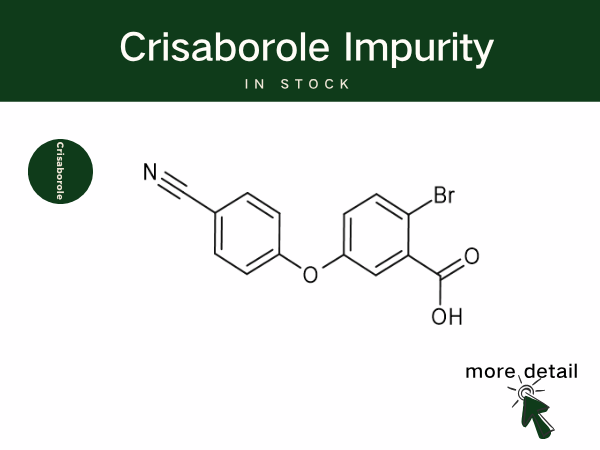
Crisaborole is Reshaping Atopic Dermatitis Treatment, Backed by SZEB Q...
Crisaborole is a boron-containing small-molecule anti-inflammatory drug, classified as a non-steroidal topical phosphodiesterase-4 (PDE-4) inhibitor. By inhibiting PDE-4, the drug increases th...
See More
-
Qilu Pharmaceutical Co.,Ltd.’s Carfilzomib for Injection Approved for M...
Qilu Pharmaceutical Co.,Ltd’s Carfilzomib for Injection has received market approval from the National Medical Products Administration (NMPA) of China for the treatment of adult patie...
See More
-
First-in-Class: FDA OKs Triple Therapy to Treat Hypertension...
Recently, George Medicines announced that its new drug Widaplik® (containing telmisartan, amlodipine, and indapamide, previously known as GMRx2) has been approved by the U.S. Food and Drug...
See More
-
LEQEMBI IQLIK™, the first-ever at-home subcutaneous injection for A...
The U.S. FDA has approved the lecanemab-irmb subcutaneous injection (U.S. brand name: LEQEMBI IQLIK™), jointly developed by Biogen and Eisai, for maintenance therapy in early A...
See More
-
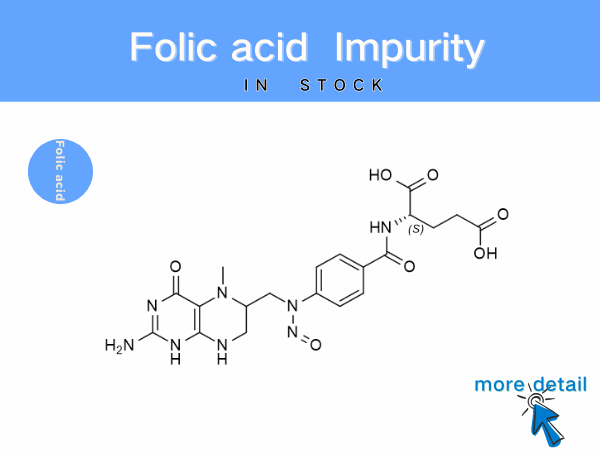
Folic Acid Impurities: Reference Standards for Pharmaceutical Quality Co...
Beyond process-related impurities, factors such as light exposure, high temperatures, or humid conditions can cause the oxidation or degradation of folic acid, leading to the formation of new impurit...
See More
-
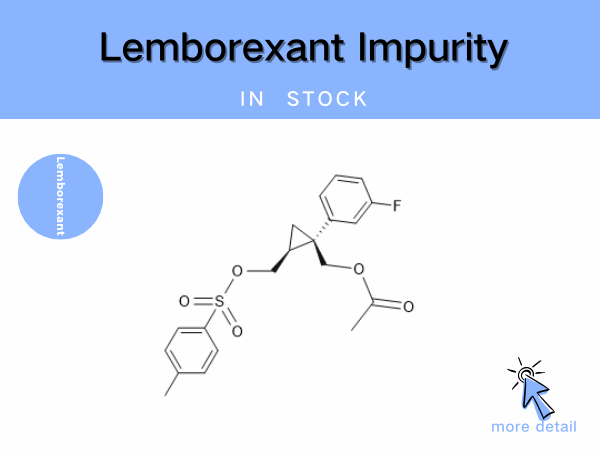
Lemborexant Impurity
Lemborexant is the first dual orexin receptor antagonist (DORA) approved for marketing in China. Developed by Eisai, it was first launched in the United States in December 2019 and received...
See More
-
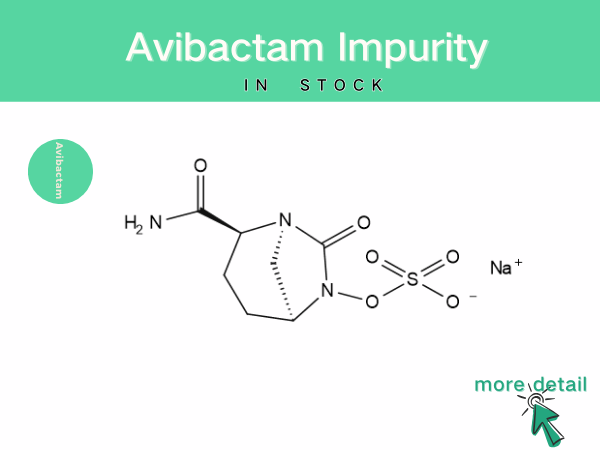
Avibactam impurity
With the widespread application and in-depth research of avibactam, the analysis and control of its impurities and content have become increasingly important. During the synthesis and production of ...
See More
-
SZEB | Continuous Updates on Nitrosamine Impurities...
Facing dynamic regulatory updates and the structural diversity of NDSRIs, Shenzhen Superior Excellence Biotechnology (SZEB) responds rapidly by supplying relevant drug impurity refer...
See More
-
Breaking Dosage Form Barriers: Escitalopram Oxalate Drops Approval ...
the Escitalopram Oxalate Drops of Guangzhou Yipinhong Pharmaceutical Co., Ltd successfully obtained the drug registration certificate issued by the National Medical Products Administration...
See More
-
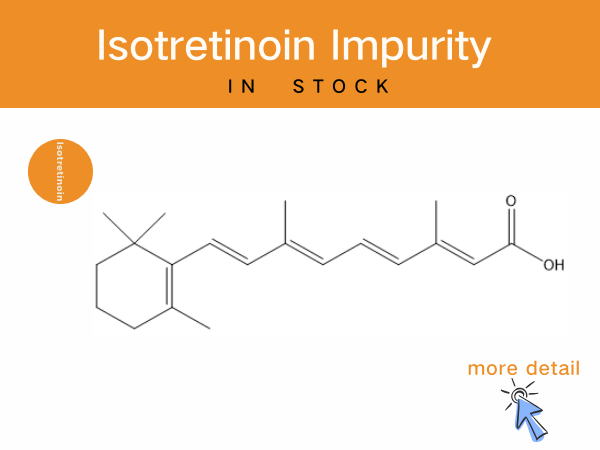
Isotretinoin Impurities: Professional Solutions of Reference Standards for...
As a critical drug for moderate-to-severe acne, Isotretinoin’s production and quality control are subject to rigorous regulatory standards. The Chinese Pharmacopoeia (2025 Edition)mandates that...
See More
-
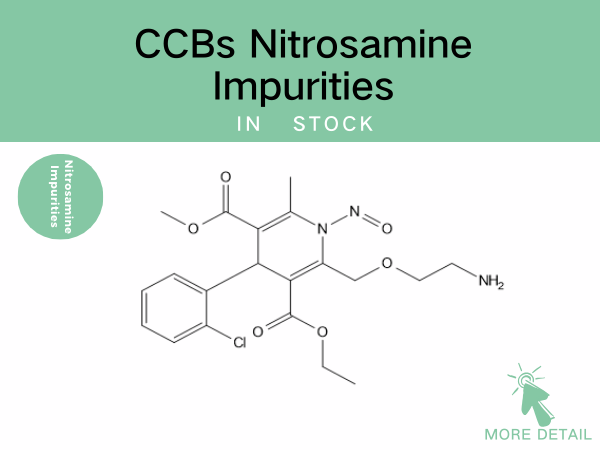
CCBs Nitrosamine Impurities——Precise Drug References Empowering Drug...
Nitrosamine impurity control remains a global regulatory priority. For CCBs—the most widely prescribed antihypertensive class—robust identification and quantification of these impurities are cr...
See More
-
the first approval of an oro-dispersible film dosage form in China...
Qilu Pharmaceutical’s risperidone oro-dispersible film has been formally approved for market launch by the National Medical Products Administration (NMPA), marking the first approval of an ...
See More












.jpg) Wechat
Wechat