Xanomeline : A Pioneering Non-Dopaminergic Therapy
The fixed-dose combination drug Cobenfy (Kar XT), composed of xanomeline and trospium chloride, received FDA approval in September 2024 for the treatment of schizophrenia in adults. This marks the first approval in decades for an antipsychotic medication with a novel, non-dopaminergic mechanism of action. Completed clinical trials have demonstrated the significant efficacy of Cobenfy in treating schizophrenia.
Traditional antipsychotics, such as chlorpromazine and clozapine, primarily exert their effects by blocking dopamine receptors and serotonin (5-HT)2A receptors, respectively.
The active ingredient in Kar XT is xanomeline, which acts as an agonist targeting the M1 and M4 subtypes of muscarinic acetylcholine receptors (M-AChRs). By activating M1 and M4 receptors in the brain, xanomeline indirectly modulates dopaminergic pathways, thereby alleviating psychiatric symptoms. This mechanism avoids the extrapyramidal symptoms and metabolic disturbances (e.g., abnormalities in glucose and lipid metabolism) commonly associated with dopamine-blocking agents.
Beyond schizophrenia, xanomeline shows promising potential in Alzheimer‘s disease treatment. Initially investigated for Alzheimer‘s, its development was halted due to significant peripheral side effects. The innovative combination of xanomeline with trospium chloride, a peripherally restricted muscarinic receptor antagonist, in the Cobenfy formulation effectively mitigates these side effects, enabling its successful application in schizophrenia. Concurrently, research indicates that Kar XT also has a beneficial effect on psychosis associated with Alzheimer‘s disease.
As further clinical trials progress, xanomeline holds promise for treating a broader range of conditions, including schizophrenia, psychosis related to Alzheimer‘s disease, and potentially bipolar disorder.
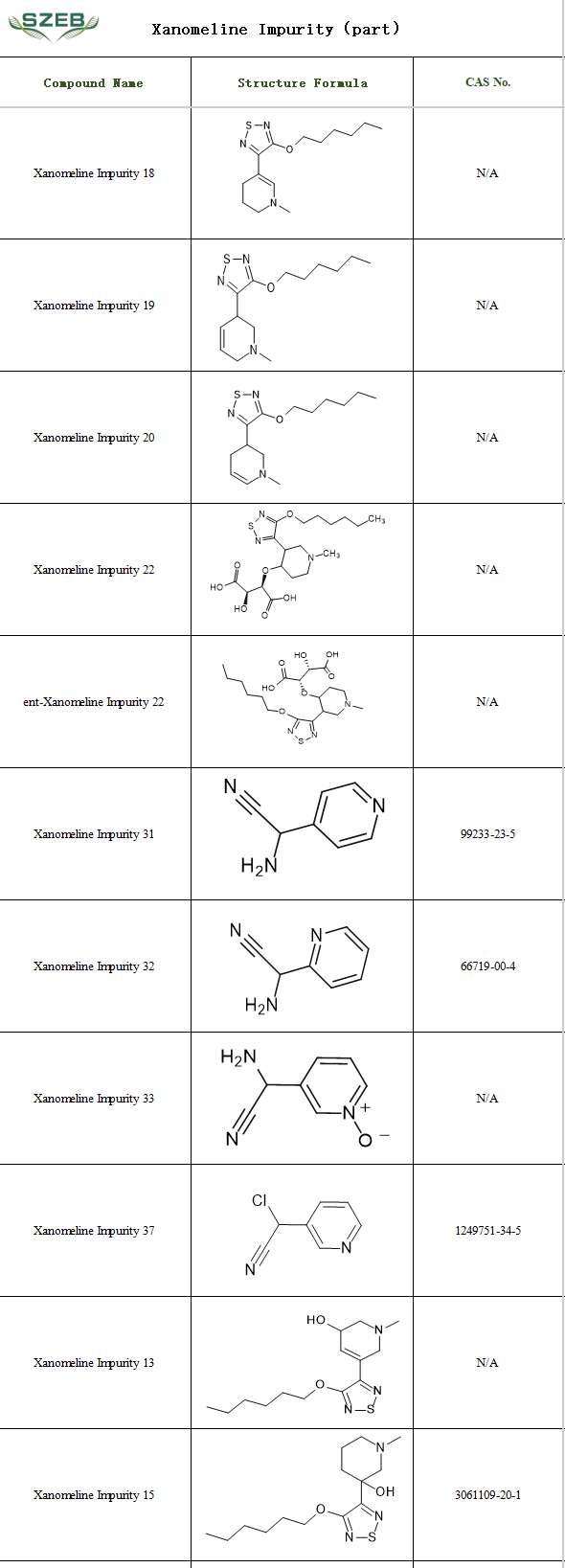
In pharmaceutical research and production, impurity profiling is a critical step to ensure drug quality and safety. Among the known impurities of xanomeline, many share structural similarities but may differ in pharmacological activity and toxicity.
Shenzhen Excellent Biomedical Technology Co., Ltd. (SZEB) supplies a comprehensive range of xanomeline impurity reference standards, providing crucial support for quality control in the development of xanomeline-based pharmaceuticals. Visit the official website at www.ex-biotech.com for more information on impurities and updates on in-stock products.






.jpg) Wechat
Wechat