-
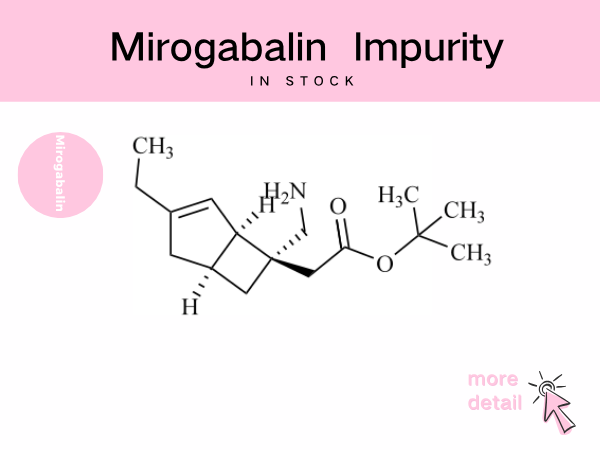
Mirogabalin Impurity ——in stock
The mirogabalin molecule contains multiple chiral centers and unsaturated bonds, rendering it susceptible to generating stereoisomers or positional isomer impurities during synthesis and storage, as ...
See More
-
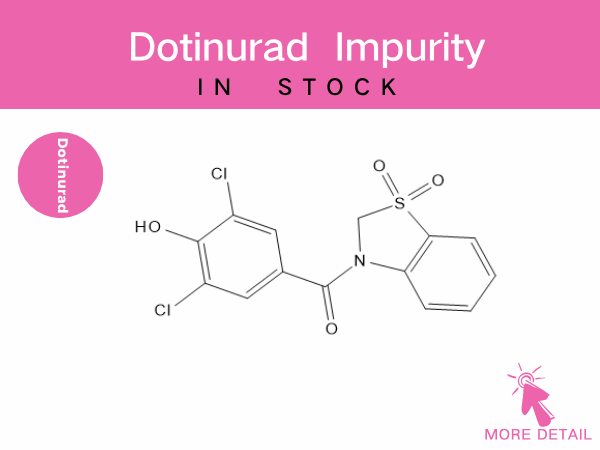
Dotinurad Related Impurities
As a novel urate-lowering drug, impurity control is a critical step in ensuring its pharmaceutical safety. Dotinurad-related impurities primarily stem from synthesis process byproducts and d...
See More
-
Mazdutide — World‘s First GCG/GLP-1 Dual-Receptor Agonist for ...
On June 27, Innovent Biologics’ Mazdutide Injection received approval from China‘s National Medical Products Administration (NMPA) for long-term weight control in adults with obesity ...
See More
-
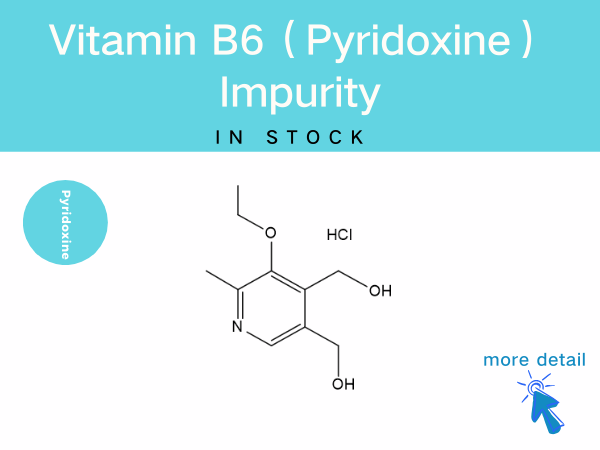
Vitamin B6 (Pyridoxine) Impurity
Vitamin B6 is not resistant to high temperatures and is easily destroyed under light and alkaline conditions, with more degradation impurities. Different impurities may also be generated during the...
See More
-
Consistency Evaluation of Generic Drugs: June 2025 Update...
In June 2025, a total of 27 varieties (39 acceptance numbers) submitted applications for consistency evaluation, 30 varieties of consistency evaluation over-evaluation, 245 deemed to have passed con...
See More
-
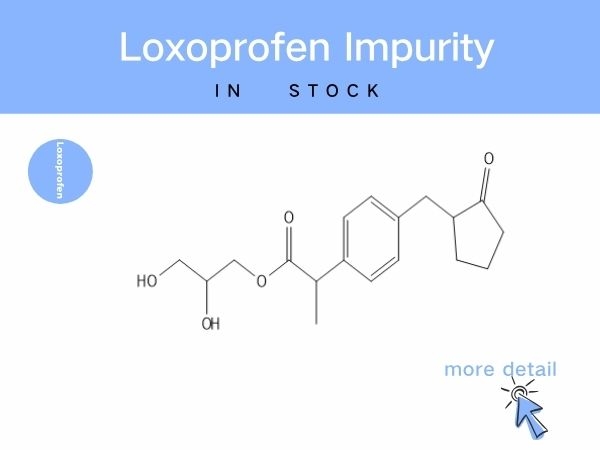
Loxoprofen Impurity
Notably, brominated intermediates from synthesis may carry potential toxicity, while impurities such as ring-opened degradants and glycerol-esterified byproducts can compromise drug e...
See More
-
Groundbreaking COPD Therapy: CTTQ PHARMA‘S TQC3721 Suspens...
CTTQ PHARMA recently announced that its inhaled PDE3/4 dual inhibitor TQC3721 suspension has received approval from China’s Center for Drug Evaluation (CDE) to initiate...
See More
-
Enhanced Supervision in Pharmaceutical Regulation...
Pharmaceutical companies must urgently implement such as stringenting raw material controls ,Enhancing process validation protocols and advancing trace-level detection capabilities for emerging carc...
See More
-
Vitamin K impurity
To address pain points in procurement, SZEB offer a full life-cycle management program: We supplies a complete range of Vitamin K impurities, including:Vitamin K1 Impurity Series...
See More
-
Finerenone impurity
SZEB supplies a comprehensive portfolio of Finerenone impurity reference standards, including stereoisomers such as: (S)-Finerenone、(R)-Finerenone、Finerenone racemate,Below i...
See More
-
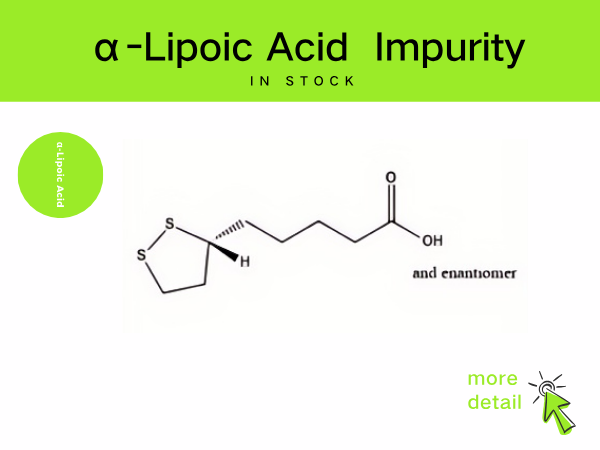
Lipoic Acid Impurity
SZEB supplies a full range of lipoic acid impurities in stock, including the main process impurities in the preparation of lipoic acid, such as: cyclization by-product impurity A , CAS: 12042...
See More
-
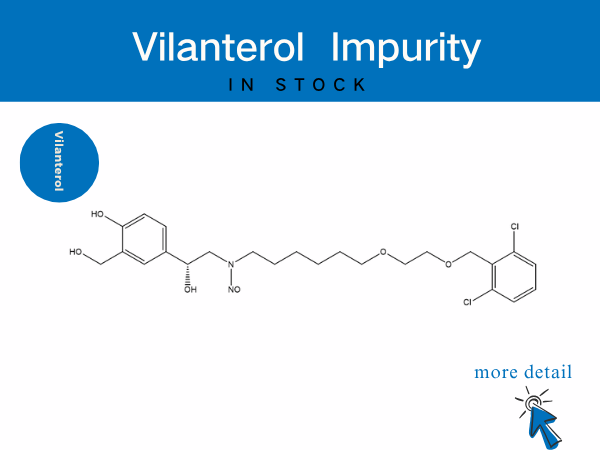
Vilanterol Impurity——in stock
SZEB provides a full suite of vilanterol impurities to accelerate generic drug bioequivalence studies and originator process optimization. Our offerings include: N-Nitroso vilanterol ...
See More
-
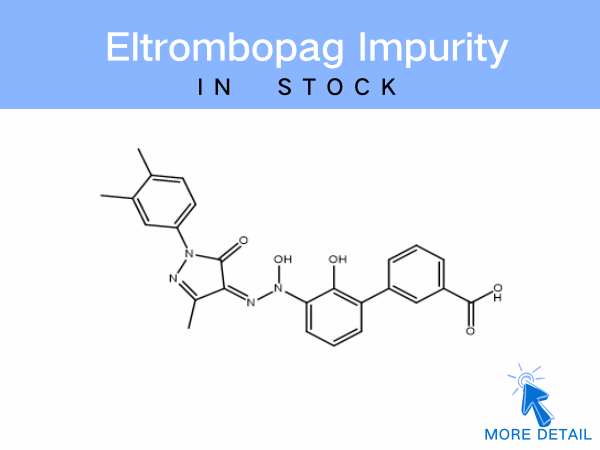
Eltrombopag Impurity
SZEB specializes in supplying a comprehensive range of eltrombopag impurities, including:EP-specified impurities、Eltrombopag olamine、Dimer-related impurities These referenc...
See More
-
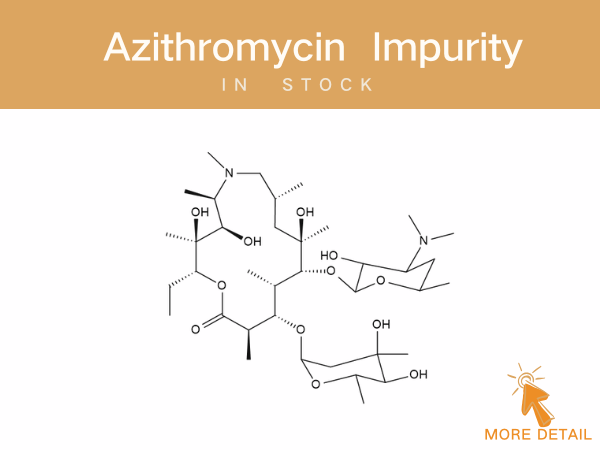
Azithromycin Impurities——in stock
Azithromycin A 11,12-cyclic borate 3’-N-Demethyl-3’-N-(phenylsulfonyl) azithromycin 3-Hydroxy azithromycin acid For detailed impurity profiles and inquiries, visit http://www.ex-biotech.c...
See More
-
AstraZeneca‘s AKT Inhibitor Capivasertib Gains Domestic Approval, Re...
The domestic launch of capivasertib propels targeted therapy advancements while necessitating more rigorous standards in multiple domains: exploration of CDK4/6 inhibitor resistance mechanisms, im...
See More
-
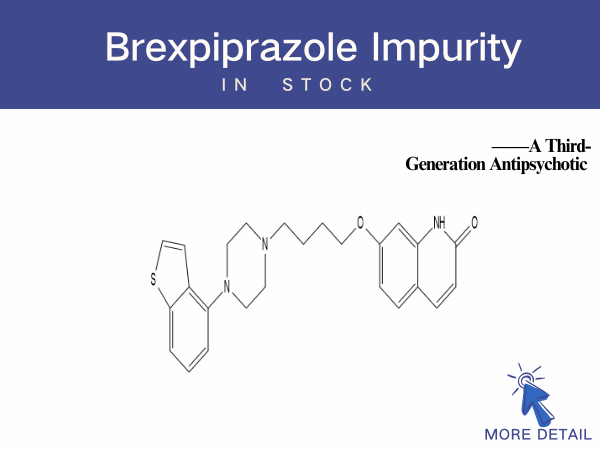
Brexpiprazole: A Third-Generation Antipsychotic...
在其实际生产工艺中,会涉及多种布瑞哌唑杂质以及相关研究分析,目前已知部分杂质包括:布瑞哌唑杂质1--50,布瑞哌唑亚砜,结构警示杂质布瑞哌唑氮氧化物等。
See More
-
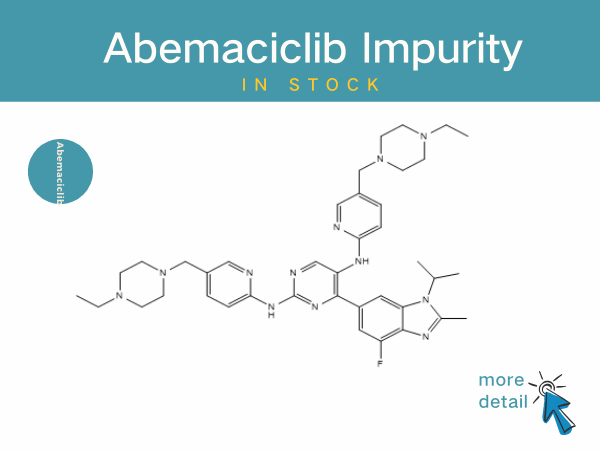
Abemaciclib impurities
Abemaciclib, the third approved CDK4/6 inhibitor worldwide, was developed by Eli Lilly and Co. It has a broad indication covering early adjuvant therapy (32% reduction in risk of recurrence) ...
See More
-
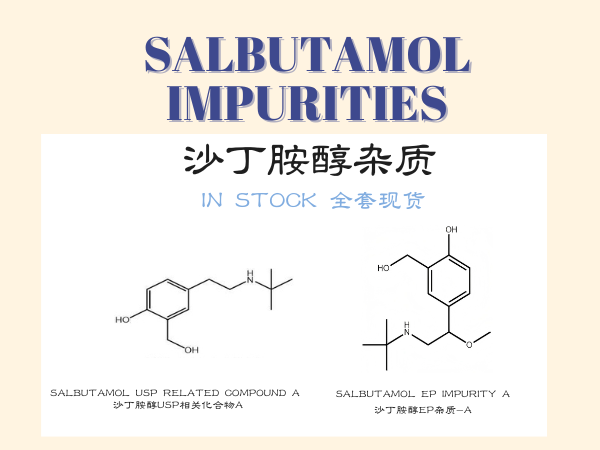
Salbutamol Impurities
Impurity control is critical to ensuring Salbutamol‘s clinical safety. Impurities may originate from synthesis processes, storage degradation, or packaging materials, and their potential t...
See More
-
Vancomycin Impurity
SZEB specializes in supplying comprehensive vancomycin impurities compliant with EP/USP standards, accompanied by COA, NMR spectra, and traceability documentation. Our services include...
See More
-
Clarithromycin Impurities
Clarithromycin, the star drug in the macrolide antibiotic class, has quickly become a go-to choice in the field of anti-infectives since its successful development by Taisho in Japan back in 1984....
See More














.jpg) Wechat
Wechat