Finerenone impurity
Time:2025-06-17
Views:169
Finerenone, as a next-generation nonsteroidal selective mineralocorticoid receptor antagonist (MRA), has emerged in recent years as a breakthrough therapeutic agent for treating type 2 diabetes-associated chronic kidney disease (T2D-CKD). Compared to traditional steroidal MRAs (e.g., spironolactone), Finerenone achieves highly selective blockade of the mineralocorticoid receptor (MR) through its unique dihydropyridine-naphthyridine heterocyclic structure. This mechanism effectively inhibits MR overactivation-mediated inflammatory responses and fibrotic processes, thereby delaying cardiorenal damage.
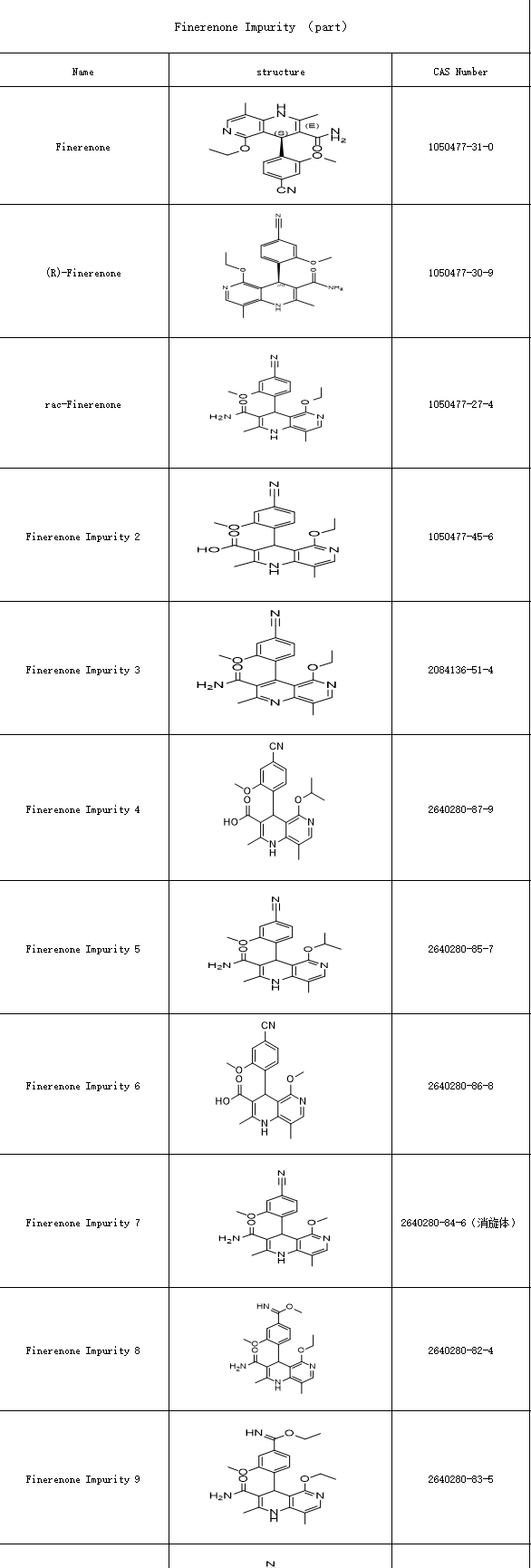
Currently, Finerenone is included in medical insurance reimbursement programs across numerous countries globally. The development of both innovator drugs and generic versions has accelerated, driving an urgent demand for Finerenone impurity research—whether for quality control of originator drugs or bioequivalence studies for generics, comprehensive impurity profiling is essential.During synthesis, process-related impurities in Finerenone may include:
Intermediate residues
Stereoisomers (e.g., the (R)-isomer)
Residual metal catalysts from synthesis.
Among degradation impurities, updated 2025 FDA guidelines explicitly list N-Nitroso-Finerenone with an acceptable intake (AI) limit of 1,500 ng/day, bringing it under regulatory scrutiny. Consequently, nitrosamine impurities have become a focal point.
SZEB supplies a comprehensive portfolio of Finerenone impurity reference standards, including stereoisomers such as:
(S)-Finerenone
(R)-Finerenone
Finerenone racemate
Below is a partial catalog of available reference materials,more information about finerenone impurity in the offical website :www.ex-biotech.com ,contact us by e-mail :sales@exbiotech.com .






.jpg) Wechat
Wechat