Mirogabalin Impurity ——in stock
Time:2025-07-28
Views:96
Mirogabalin, marketed as Mirogabalin Besilate Tablets (Trade Name: Tarlige®), is the first and only imported originator drug approved in China for the treatment of Diabetic Peripheral Neuropathic Pain (DPNP) in adults.
Clinical Significance of DPNP
Diabetic Peripheral Neuropathic Pain (DPNP), one of the most prevalent chronic complications of diabetes, manifests primarily as sensory abnormalities and pain in the extremities (particularly bilateral feet). Symptoms include burning pain, needle-like/electric shock-like pain, tearing/lancinating pain, numbness/hypoesthesia, abnormal pain sensitivity (allodynia), along with motor and autonomic dysfunction. Nocturnal exacerbation of pain significantly impairs sleep quality and, long-term, predisposes patients to mood disorders and functional decline. Epidemiological studies indicate over 20 million DPNP patients in China, highlighting an urgent unmet therapeutic need.
Pharmacological Profile
As a next-generation calcium channel modulator, mirogabalin exerts analgesic effects by selectively binding to the α2-δ subunit of voltage-gated calcium channels in the central nervous system. This action reduces the release of excitatory neurotransmitters (e.g., glutamate), thereby inhibiting pain signal transduction and alleviating chronic neuropathic pain (e.g., limb numbness, tingling, or burning sensations) induced by diabetic peripheral neuropathy. Studies suggest mirogabalin may enhance the activity of descending noradrenergic pathways, activating endogenous pain-inhibitory systems to potentiate analgesic efficacy. Distinguished from conventional analgesics (e.g., pregabalin, gabapentin), mirogabalin demonstrates superior efficacy and safety, establishing itself as a preferred therapy for DPNP—particularly in patients requiring long-term medication, those with renal impairment, or those intolerant to first-line agents.
Quality Control Challenges
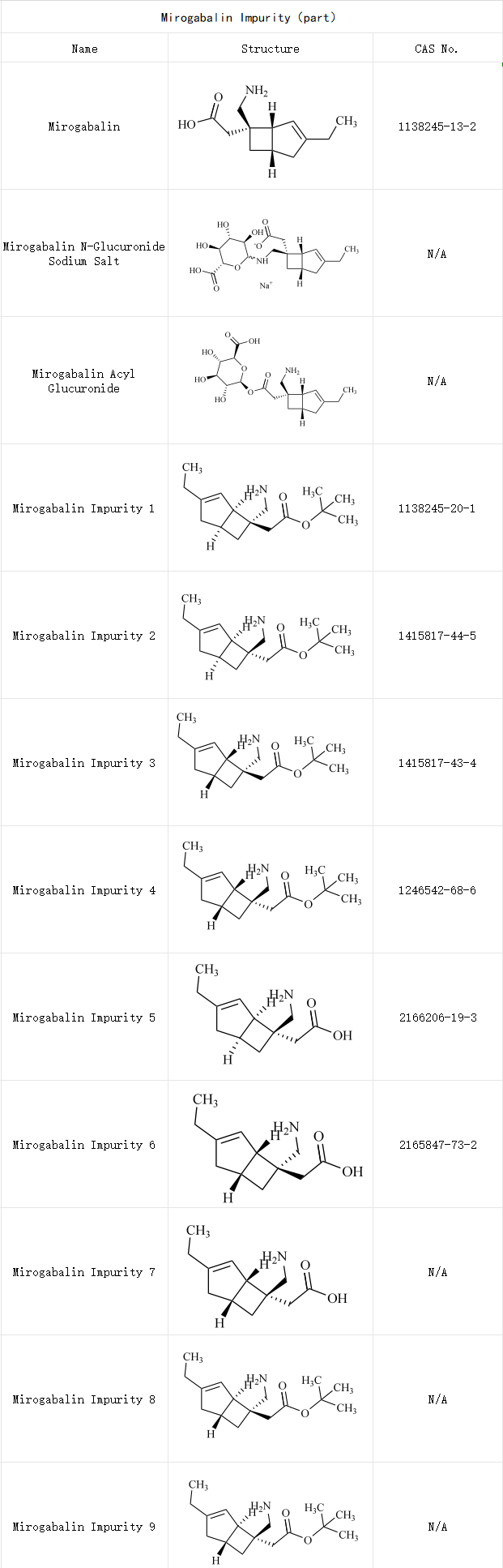
Current clinical data and related research on this novel agent remain limited, making stringent quality control strategies paramount to ensuring its safety and efficacy. Impurity profiling constitutes a critical component of this framework. The mirogabalin molecule contains multiple chiral centers and unsaturated bonds, rendering it susceptible to generating stereoisomers or positional isomer impurities during synthesis and storage, as well as degradation products such as lactam impurities and oxidative ring-opening impurities. Effective impurity control necessitates integrated analysis of synthetic pathways and chemical properties, with particular emphasis on genotoxic impurities and irreversible degradation products.
Mirogabalin Impurity References Support
SZEB specializes in comprehensive solutions for pharmaceutical impurity references. We supply mirogabalin impurity references to support pharmaceutical enterprises and research institutions in impurity characterization studies. Partial catalog listed below;
Moere details available at: http://www.ex-biotech.com
Inquiry Hotline: 0755-23051186 .






.jpg) Wechat
Wechat