‘Parkinson‘s’ drug development - pramipexole impurities
Pramipexole is a dopamine receptor agonist that binds with high selectivity and specificity to the D2 subfamily of dopamine receptors and has full intrinsic activity, with preferential affinity for the D3 receptor therein. Pramipexole attenuates dyskinesia in Parkinson‘s patients by excitation of dopamine receptors in the striatum.
The sources of impurities in pramipexole can be categorised into several:
One is impurities from the synthesis process. For example, the pramipexole hydrochloride degradation impurity BI-IO460BS is obtained by a series of chemical reactions via pyroglutaminol. In addition, three impurities of pramipexole dihydrochloride can also be generated by Mitsunobu reaction.
Secondly, impurities of plant origin: the raw materials of pramipexole may come from different plant families, such as Ledebouriaceae, Hydrocotyledonaceae, Nannochloraceae, etc., and the impurities in these plants may get into the final product.
In the synthetic study of pramipexole hydrochloride and its related impurities, the methods to effectively control the impurity content and ensure the quality and safety of the drug mainly include the following aspects:
Raw material handling: selecting raw materials with high purity. For example, benzoic anhydride needs to be refined and dried to ensure the purity and stability of the reaction.
Optimisation of reaction conditions: Yield and purity can be significantly improved by optimising parameters such as solvent, reaction temperature and feed ratio. For example, it has been shown that adjusting the termination temperature of precipitation and the rate of refinement cooling has a significant effect on yield.
Selection and use of catalysts: Selection of suitable chiral catalysts and their use under appropriate conditions can improve the selectivity of the reaction and thus reduce the generation of impurities. For example, the condensation and reduction reactions in organic solvents in the presence of chiral catalysts can give pramipexole in one pot.
Comprehensive process flow: Through systematic research on the synthesis process, reaction conditions and product separation and purification methods, a complete set of synthetic process flow can be formed, which can be used as a basis to further improve the yield and purity and provide better raw materials for the pharmaceutical industry.
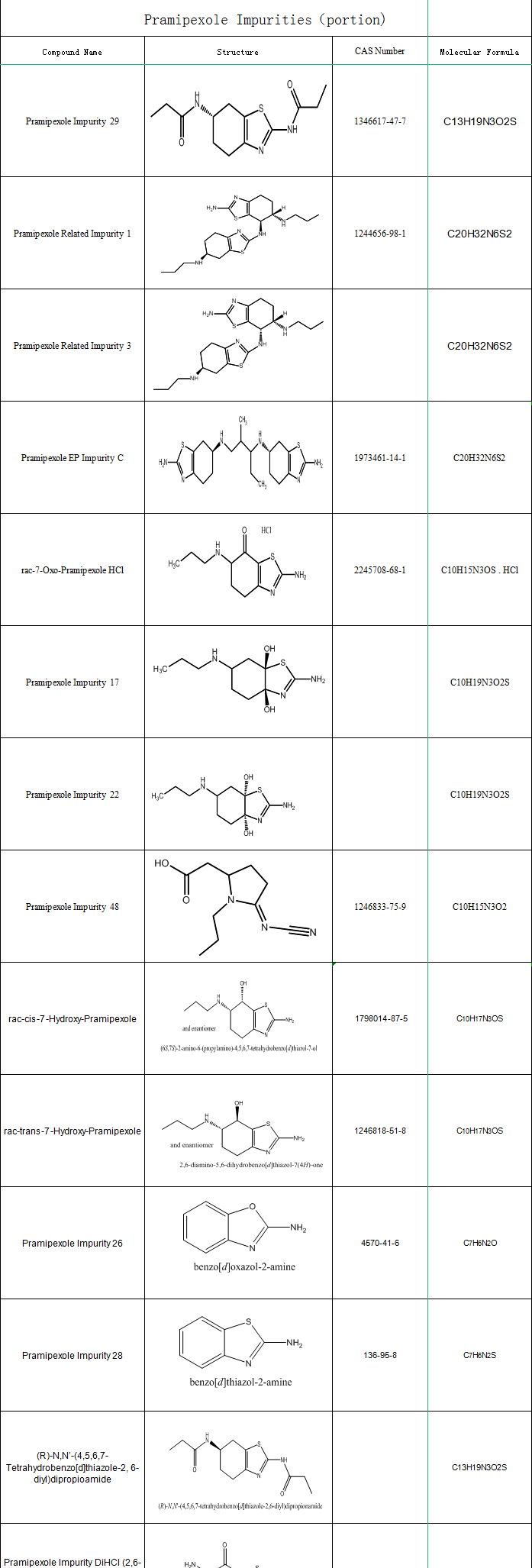
SZEB supplies pramipexole impurityties, the following is part of the pramipexole impurities catalogue, more impurity information please go to the official website: https://www.ex-biotech.com. You can call us on: 0755-23051186 / 0755-26050679






.jpg) Wechat
Wechat