Update to living WHO guideline on drugs for covid-19
Time:2022-04-26
Views:692
The World Health Organization (WHO) has released the ninth update of their living guideline on medications for the treatment of COVID-19 infection. These updates were published in BMJ (A living WHO guideline on drugs for covid-19).
In this update the Guideline Development Group (GDG) developed new recommendations for patients with non-severe covid-19, concerning the use of nirmatrelvir/ritonavir and remdesivir.
New recommendation

In this update the Guideline Development Group (GDG) developed new recommendations for patients with non-severe covid-19, concerning the use of nirmatrelvir/ritonavir and remdesivir.
New recommendation
• Strong recommendation for the use of nirmatrelvir-ritonavir in patients with non-severe illness at the highest risk of hospitalization.
• Conditional recommendation against the use of nirmatrelvir-ritonavir in patients with non-severe illness at a low risk of hospitalization.
• Conditional recommendation for the use of remdesivir in patients with non-severe COVID-19 at the highest risk of hospitalization.
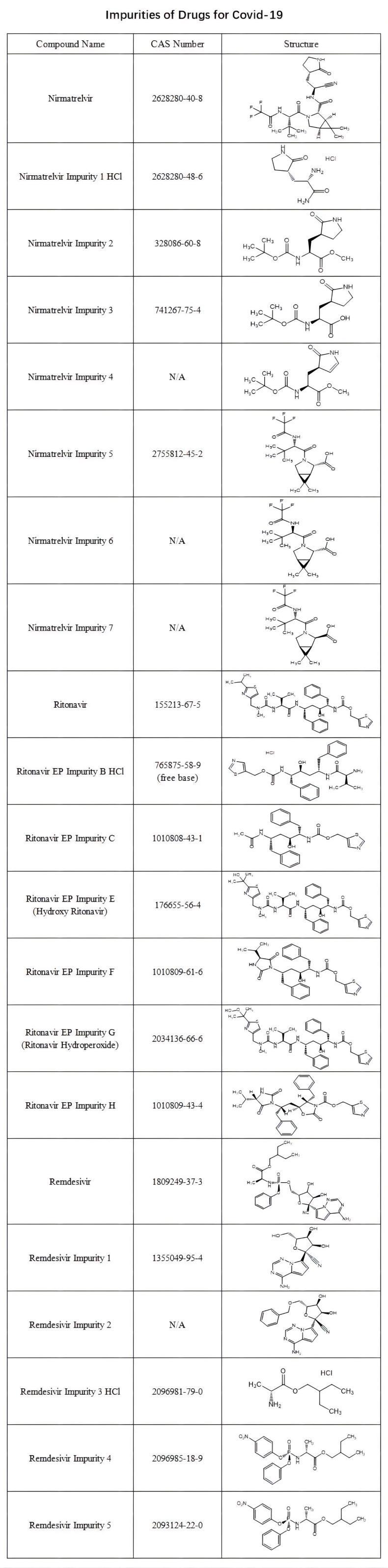
To support your research and analysis of drugs for covid-19, we supply a range of nirmatrelvir-related impurities, ritonavir-related impurities and remdesivir-related impurities standards with certified COA and characterization data like Mass, HPLC, NMR & TGA report.
Discover all our products through our official website, and get in touch if you have any questions about how we can help support your work. Email:sale@ex-biotech.com.
To support your research and analysis of drugs for covid-19, we supply a range of nirmatrelvir-related impurities, ritonavir-related impurities and remdesivir-related impurities standards with certified COA and characterization data like Mass, HPLC, NMR & TGA report.
Discover all our products through our official website, and get in touch if you have any questions about how we can help support your work. Email:sale@ex-biotech.com.







.jpg) Wechat
Wechat