Azithromycin Impurities——in stock
Time:2025-04-30
Views:2
As a second-generation macrolide antibiotic, azithromycin was developed in 1980 by Pliva (Croatia) and later approved in the U.S. by Pfizer in 1991 under the brand name Zithromax®, rapidly becoming a blockbuster drug in global anti-infective therapy. China officially approved the import of the originator drug in 2021, while domestic generic versions entered the market as early as 1995. Current formulations of azithromycin in China include tablets, capsules, injectables, and dry suspensions, addressing diverse clinical needs.
Leading pharmaceutical enterprises dominate the market through scale advantages, with product portfolios spanning from active pharmaceutical ingredients (APIs) to high-end formulations, while small-to-medium manufacturers focus on niche segments such as pediatric formulations and sustained-release preparations.
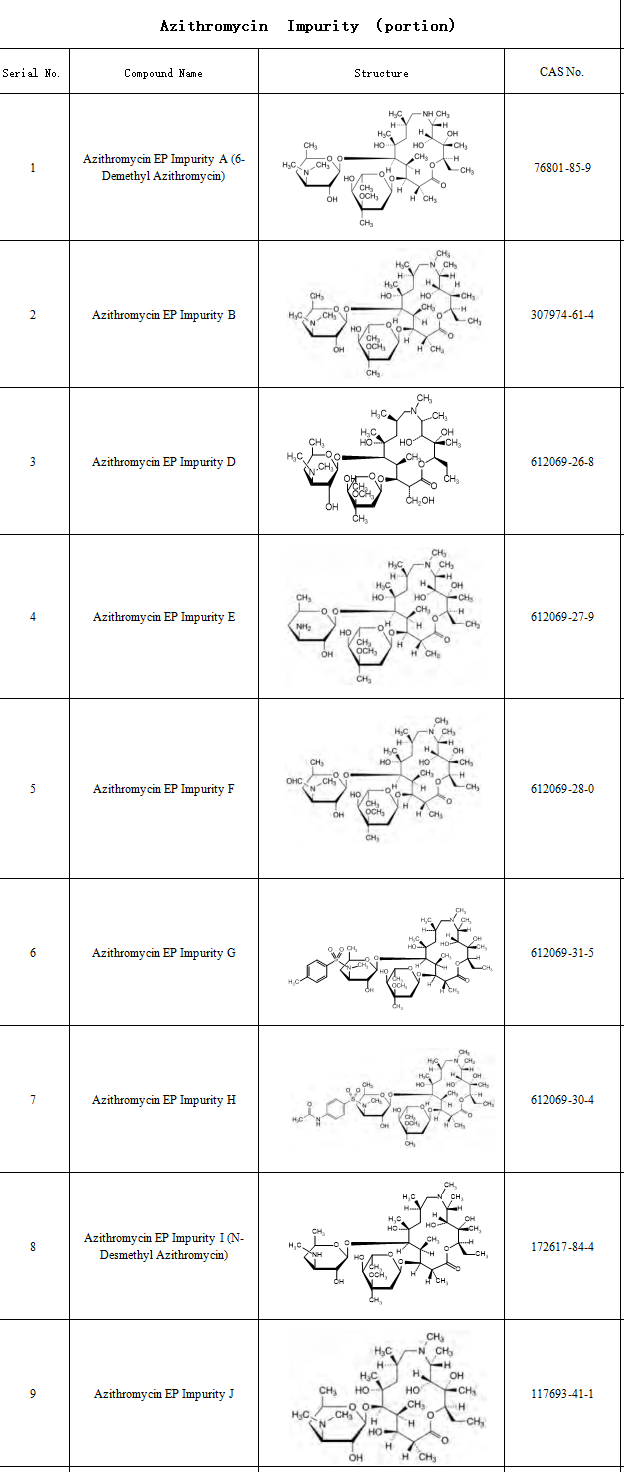
Impurity control critically impacts drug safety and stability. Azithromycin’s complex synthesis—involving oximation, molecular rearrangement, and ring expansion—generates multiple process-related impurities and degradation products. Storage conditions (e.g., acidic/alkaline environments) may also induce degradation; for instance, citric acid as a solubilizing agent can lead to impurity exceedances. Regulatory requirements under the Chinese Pharmacopoeia (ChP) and EMA guidelines have tightened impurity controls, specifying a 0.5% threshold for individual azithromycin impurities.
To address research challenges in impurity profiling and quality control, SZEB provides comprehensive solutions. We specialize in supplying a complete range of azithromycin impurity reference standards compliant with EP/USP pharmacopeial specifications, including:
Azithromycin A 11,12-cyclic borate
3’-N-Demethyl-3’-N-(phenylsulfonyl) azithromycin
3-Hydroxy azithromycin acid
For detailed impurity profiles and inquiries, visit http://www.ex-biotech.com or contact our sales team at +86-0755-23051186.






.jpg) Wechat
Wechat